thyssenkrupp AG – Annual report – 30 September 2021
Industry: manufacturing; steel
Disposal groups
In the course of its transformation and strategic realignment, thyssenkrupp is also focusing on its portfolio. In this context in the 4th quarter of 2020 / 2021 the disposal of Mining, Infrastructure and the stainless steel business has been initiated. These transactions do not meet the requirements of IFRS 5 for a presentation of a discontinued operation. Therefore, expenses and income will continue to be presented as income from continuing operations until the date of disposal and the assets and liabilities of the respective disposal groups will be disclosed separately in the balance sheet as of September 30, 2021 in the line items “Assets held for sale” and “Liabilities associated with assets held for sale”.
Disposal group Mining
On July 29, 2021, thyssenkrupp signed an agreement to sell the mining business in the Multi Tracks segment to Danish company FLSmidth. The disposal group provides technologies for the mining industry. The transaction is subject to merger control approval. Signing triggers extensive carve-out activities expected to be completed by closing in a period of approximately twelve months. In connection with the initiated sale immediately before the initial classification as a disposal group it has been ensured that the measurement of the assets is in accordance with IAS 36; this has not resulted in any impairment. The assets and liabilities of the disposal group as of September 30, 2021 are presented in the following table; €(30) million of cumulative other comprehensive income presented within equity is attributable to the disposal group.

Disposal group Infrastructure
On August 5, 2021, thyssenkrupp signed an agreement with FMC Beteiligungs KG to sell Infrastructure in the Multi Tracks segment. The disposal group is active in civil engineering, port engineering and special-purpose civil engineering, as well as in structural engineering. The product portfolio comprises the areas of profiles and anchor technology, flood protection, pile driving and drawing technology, drilling engineering, trench sheeting and shoring. Closing is expected in the second half of calendar year 2021.
In connection with the allocation of Infrastructure to the Multi Tracks segment, comprehensive studies of the market environment and of the potential for disposing of individual assets were performed. In light of this, Infrastructure was tested again for impairment under IAS 36 in the 3rd quarter of 2020 / 2021 and an impairment loss of €27.3 million was identified; €0.2 million of this amount related to intangible assets and €24.3 million to property, plant and equipment. €2.8 million could not be recognized due to the lower value limits under IAS 36.105. The relevant recoverable amount used to determine the impairment loss in each case corresponded to the respective value in use, which amounted to €58 million in total and was determined applying a discount rate (after taxes) of 7.65%. In addition, the carrying amounts of assets and liabilities were reviewed in the 4th quarter of 2020 / 2021 on initial classification as a disposal group as part of the initiated sale process; this resulted in further impairment losses of €20 million, which relate in particular to current assets. Impairment losses are reported in cost of sales in the 4th quarter of 2020 / 2021. Following the recognized impairment losses, the carrying amount of the disposal group corresponds to fair value less costs of disposal.
The assets and liabilities of the disposal group as of September 30, 2021 are presented in the following table; €(2) million of cumulative other comprehensive income presented within equity is attributable to the disposal group.

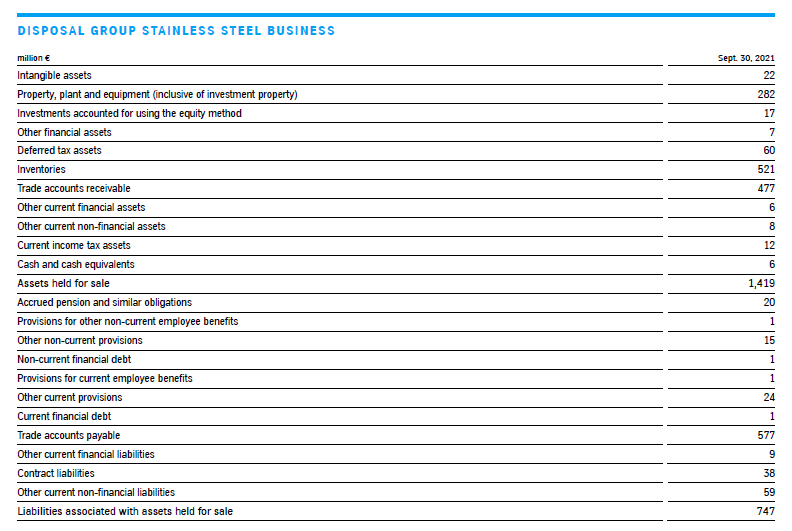
Disposal group stainless steel business
On September 16, 2021, thyssenkrupp signed an agreement with Arvedi Group, Italy, to sell the stainless steel business (stainless steel plant in Terni, Italy, (AST) including the associated sales organization in Germany, Italy and Turkey) from the Multi Tracks segment. Among other things, the transaction is subject to merger control approval. Closing of the sale is expected in the first half of 2022. thyssenkrupp is also examining a potential minority investment in AST; the details will be negotiated prior to closing.
The carrying amounts of the assets and liabilities were reviewed in connection with the initiated sale process on initial classification as a disposal group in accordance with IAS 36; this resulted in the reversal of an impairment loss in the total amount of €38 million, as the fair value (level 3, derived from the purchase price) less costs of disposal is higher than the carrying amount. This had been written down as of September 30, 2020, due to the lower expectations regarding the future results of operations because of the coronavirus pandemic. Of the total €38 million reversal, €6 million is attributable to buildings and €32 million to technical machinery and equipment. It is reported in cost of sales in the 4th quarter of 2020 / 2021; deferred tax liabilities of €11 million were recognized at the same time.
The assets and liabilities of the disposal group as of September 30, 2021 are presented in the following table. There were also intragroup financing liabilities of €276 million as of the reporting date. €(1) million of cumulative other comprehensive income presented within equity is attributable to the disposal group.